A multi-institutional research team led by scientists at the AIDS Institute and Department of Microbiology, LKS Faculty of Medicine of The University of Hong Kong (HKUMed) has identified BilA-SG, a tandem bi-specific broadly neutralising antibody, as a promising universal drug for human immunodeficiency virus-1 (HIV-1) prevention and functional cure. A single pre-exposure intramuscular injection of BilA-SG was found to be 100% effective in preventing a pathogenic virus and a similar post-exposure treatment sustained 100% survival and complete virus suppression in most of the non-human primate models for two years. The new findings are now published in the August issue of Cell Reports, one of the world’s leading scientific journals.
Background
This year marks the 40th anniversary of the incurable AIDS, which has caused about 40 million deaths to date globally, and 37.6 million people living with HIV globally. To end the HIV/AIDS pandemic, it is important to discover either an effective vaccine or a therapeutic cure. However, the tremendous HIV-1 diversity and the antiviral drug-unreachable latency remain the greatest two challenges. Since it is still unsuccessful to develop an appropriate immunogen to elicit broadly neutralising antibodies (bnAbs) against genetically divergent HIV-1 subtypes, the HKUMed research team has invented the BiIA-SG as passive immunisation for HIV-1 prophylaxis and immunotherapy.
The team has previously investigated the potency and breadth of BiIA-SG in both in vitro and humanised mice. By attaching to host protein CD4, BiIA-SG strategically ambushes invading HIV-1 particles to protect CD4 positive T cells. BiIA-SG not only displays a potent and universal activity against all three panels of 124 genetically divergent global HIV-1 strains tested, but also prevents diverse live viral challenges completely in humanised mice. Moreover, gene transfer of BiIA-SG achieves prolonged drug availability in vivo, leading to a promising efficacy of eliminating HIV-1 infected cells in humanised mice. These results provide a proof-of-concept that BiIA-SG is a novel universal antibody drug for prevention and immunotherapy against HIV-1 infection.
Research method and findings
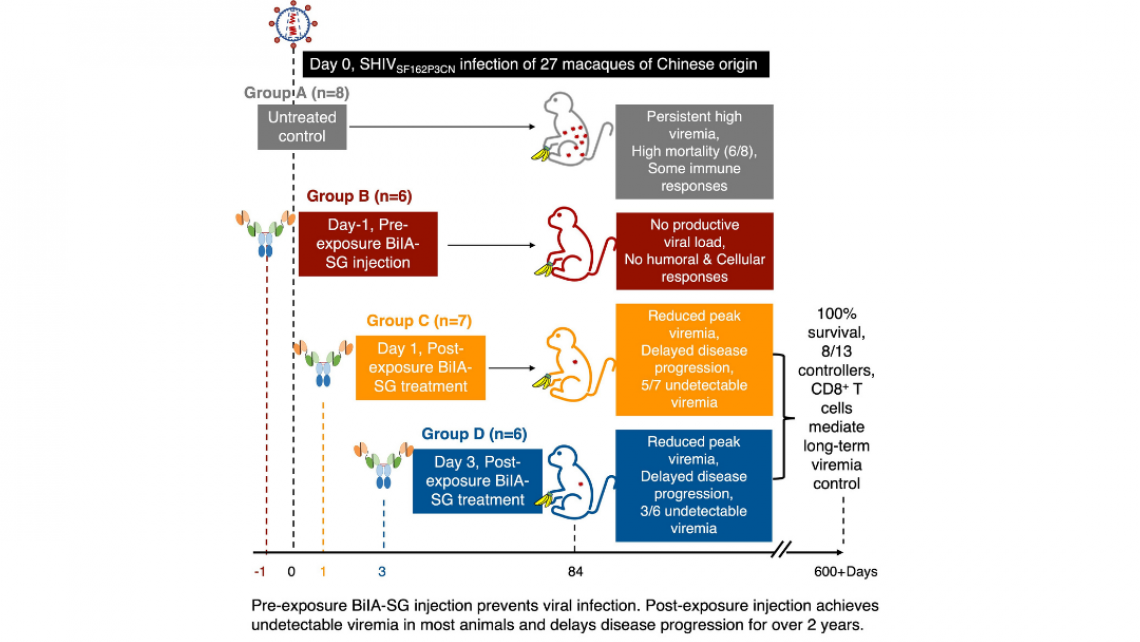
In this study, using a rhesus monkey model of simian-human immunodeficiency virus (SHIVSF162P3CN) infection that resembles properties of HIV, the research team found that intravenous SHIVSF162P3CN challenge resulted in high mortality as six of eight untreated rhesus monkeys had developed simian AIDS within two years. In contrast, single pre-exposure intramuscular BiIA-SG injection prevented viral infection in all six monkeys with neither measurable virus nor host immune responses to the virus. Moreover, after viral infection, early treatment by a single intramuscular BiIA-SG injection has reduced peak viremia in 77% of the animals, achieves undetectable setpoint viremia in 61.5% of the monkeys, and delays disease progression with 100% survival for two years in all treated animals. BiIA-SG therapy-induced CD8 T cells contribute to viral suppression in controller animals. These results, therefore, warrant BiIA-SG to be developed as a novel universal antibody drug for prevention and immunotherapy against HIV-1 infection.
Significance of the study
The combination antiretroviral therapy (cART) introduced in 1995 has greatly reduced AIDS-related mortality. However, since cART is a life-long regiment, the treatment cost remains an unsustainable financial burden to the 37.6 million people infected with HIV, in addition to the increasing issues of accumulative drug toxicity and resistant viruses. The discovery of a functional cure, defined by sustained unmeasurable viral load in the absence of cART, will have significant clinical benefits to patients and pandemic control.
Besides BiIA-SG, the same HKUMed research team has recently demonstrated the sustained viremia suppression through induction of potent effector-memory CD8+ T cells by a PD1-based AIDS vaccine in rhesus monkeys as published recently in the June Issue of PLoS Pathogens. ‘By developing BiIA-SG and the PD1-based AIDS vaccine clinically, we aspire to generate the first “Made-in-Hong Kong” immunotherapy for HIV/AIDS functional cure,’ said Professor Chen Zhiwei, Director of AIDS Institute and Professor of Department of Microbiology, HKUMed, who led the research.
About the research team
The research was conducted primarily by the HKUMed team led by Professor Chen Zhiwei, Director of the AIDS Institute and Professor of Department of Microbiology, HKUMed. Former graduate student Dr Niu Mengyue and post-doctoral fellow Dr Wong Yik-chun of Department of Microbiology, HKUMed, Dr Wang Hui of Shenzhen Third People’s Hospital and Foshan University graduate student Miss Li Xin made equal contributions. Key external collaborators also included Professor Zhang Haoji of Foshan University, Professor Jin Xia of Fudan University, and Professor Zhang Linqi of Tsinghua University.
Acknowledgements
The work was supported by the Hong Kong Research Grants Council (RGC) Theme-based Research Scheme (T11-706/18-N), the French National Research Agency (ANR)/RGC Joint Research Scheme (A-HKU709/14), General Research Fund (762712) and Collaborative Research Fund (HKU5/CRF/13G); China’s National Science and Technology Major Project (2017ZX10201101-004); Health and Medical Research Fund (17160762) of Food and Health Bureau, Government of the Hong Kong Special Administrative Region; the Sanming Project of Medicine in Shenzhen; HKU University Development Fund and the Li Ka Shing Faculty of Medicine Matching Fund to the AIDS Institute, HKUMed.
About the AIDS Institute, HKUMed
Established in 2007, the AIDS Institute, LKS Faculty of Medicine of The University of Hong Kong (HKUAI) has a long and distinguished history in HIV/AIDS education and research. Currently, HKUAI is leading the undergraduate course of Infection and Immunity for the Bachelor of Biomedical Sciences programme. HKUAI is also leading the Theme-Based Research Scheme on HIV-1 Vaccine and Cure granted by the Hong Kong Research Grants Council. HKUAI has made significant contributions through its research and advocacy to improve the health of populations and individuals in the fight against the AIDS pandemic, which led to the winning of 2019 Knowledge Exchange Awards of LKS Faculty of Medicine and The University of Hong Kong, respectively.
To promote knowledge exchange, research activities at HKUAI can be viewed through med.hku.hk/aidsinst.