Despite their popular use today, lithium-ion batteries have the drawbacks of being toxic and expensive, with a global supply shortage of metal. And for decades, researchers have tried to look for alternatives which are more environmental-friendly, safer, and of lower cost.
A team of researchers led by Professor Dennis Leung from the Department of Mechanical Engineering at the University of Hong Kong (HKU) has discovered a new possibility – a rechargeable aqueous battery with a magnesium metal anode. The innovation opens a new direction for the development of post-lithium-ion batteries.
The team’s findings, which were published in ACS Energy Letters, in an article titled “Reversibility of a high-voltage, Cl-assisted, aqueous Mg metal battery enabled by a water-in-salt electrolyte”, bring attention to the overlooked rechargeable aqueous Magnesium (Mg) metal batteries.
“With a high theoretical capacity and negative electrochemical potential, magnesium is an attractive anode material,” said Professor Leung. “Magnesium is also non-toxic and earth-abundant.”
Mg makes up over 2% of the earth’s crust, which is more abundant than lithium by 1000 times. For long, Mg metals were considered hard to be used in batteries because of their high reactivity. Mg is passivated when exposed to moisture, forming an impermeable oxidation film that blocks redox reactions. Most researchers study Mg batteries with non-aqueous organic electrolytes, but they are often costly, unstable, and poorly conductive.
Professor Leung maintains that aqueous electrolytes do offer a safe and low-cost solution, despite the challenge posed by magnesium’s sensitivity to moisture. “It would make a promising candidate for low-cost and sustainable batteries if we can unlock the potential of aqueous Mg batteries.”
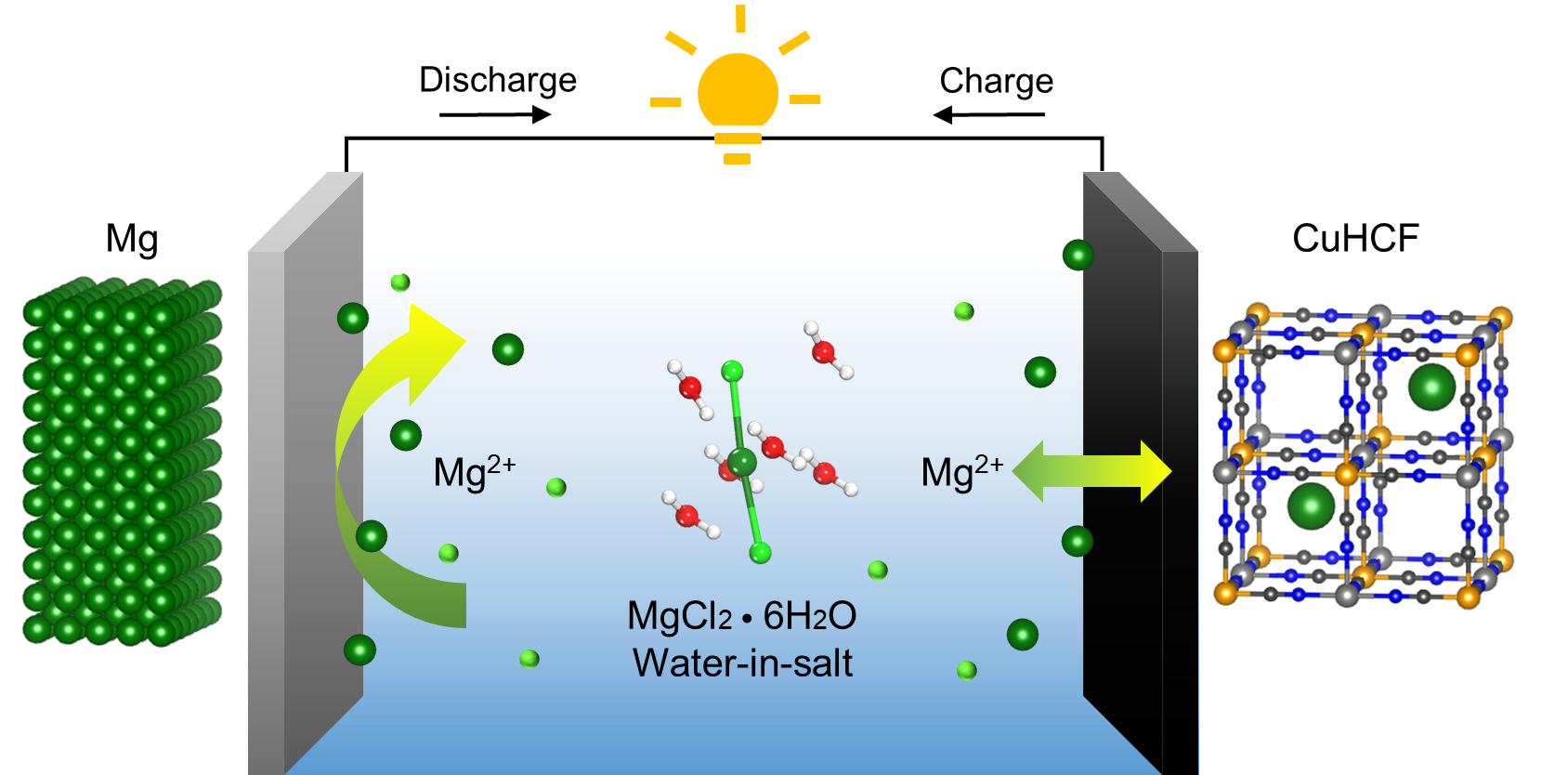
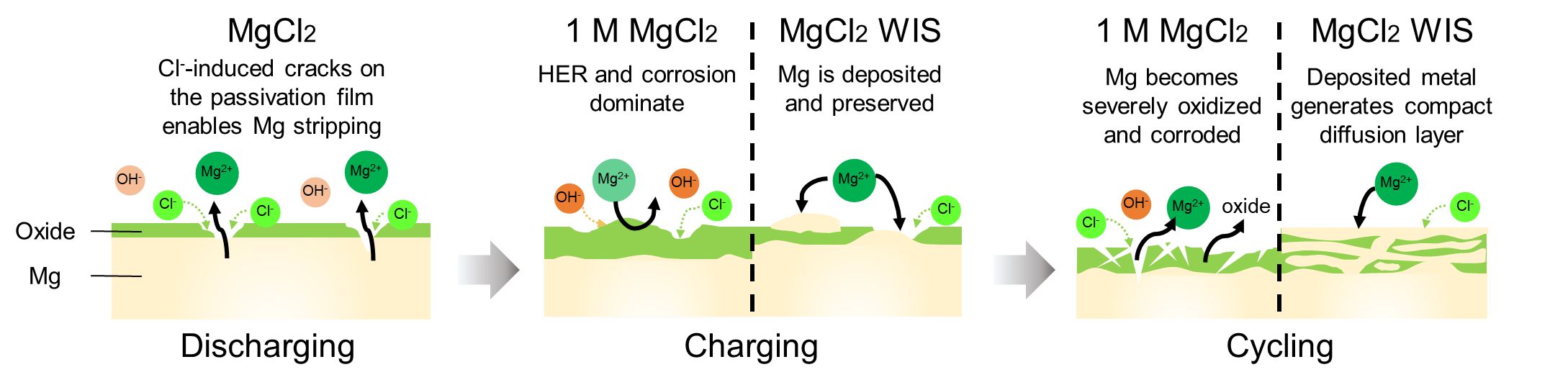
And that is what his team has discovered. They found that contrary to traditional belief, rechargeability can be achieved in an aqueous Mg battery system. The Mg passivation film can be regulated using an aqueous chloride-based “water-in-salt” electrolyte.
A “water-in-salt” electrolyte is a supersaturated mixture where the mass of solute outweighs that of the solvent. “The limited availability of free water in the water-in-salt electrolyte restricts water decomposition and addresses the main cause of passivation,” explained Dr Wending Pan, a postdoctoral fellow from the Department of Mechanical Engineering who specializes in the study of water-in-salt electrolytes.
The team also found that the adsorption of chloride ions can protect the Mg surface by partially dissolving oxides and exposing native metal for redox reactions. With limited free water, the chloride-based water-in-salt electrolyte successfully combats magnesium passivation.
“Using the novel water-in-salt electrolyte, the original passivation film can be converted into a conductive metallic oxide layer, providing ionic pathways for rechargeable battery operations,” said PhD student Kee Wah Leong, who studied the surface of the magnesium anode in detail.
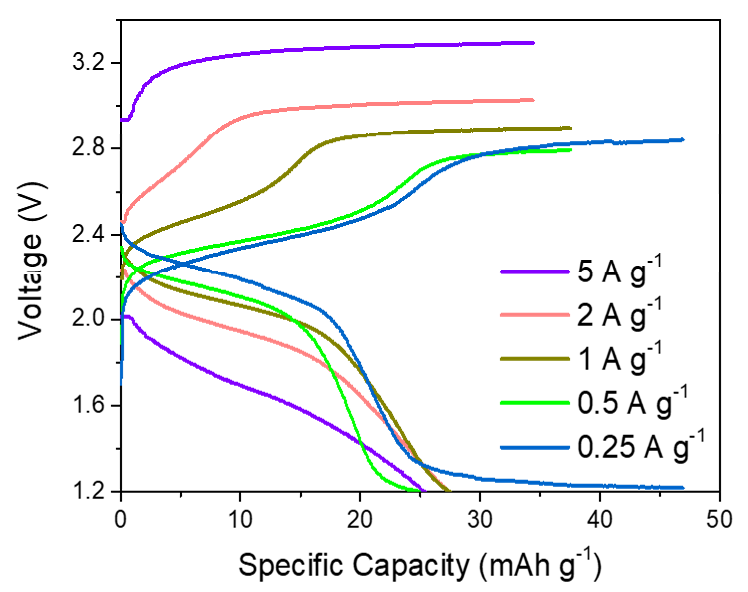
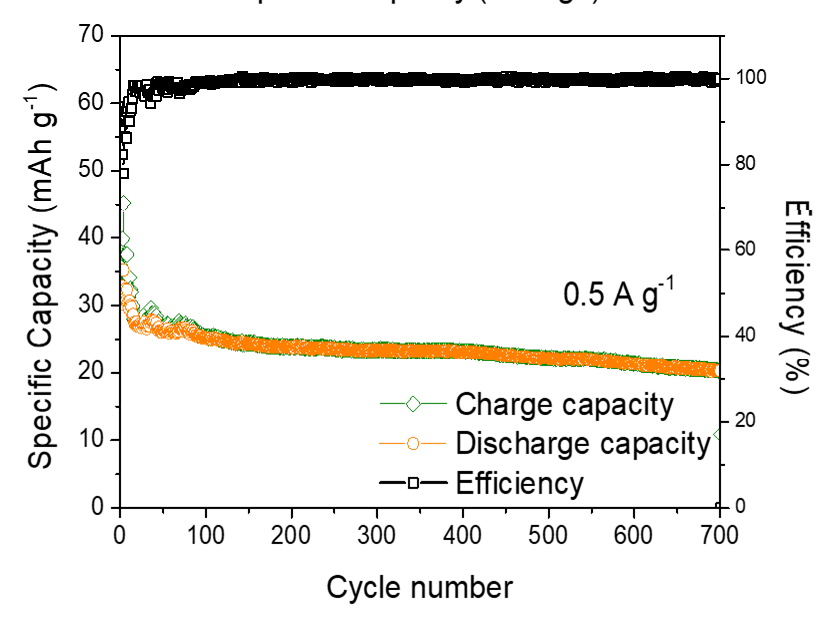
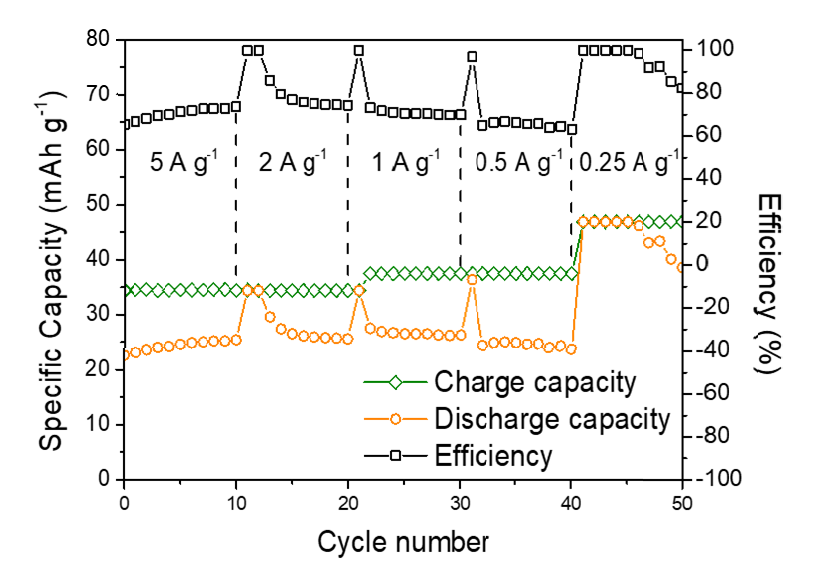
The resulting battery demonstrates excellent rechargeability for over 700 stable cycles with a high discharge plateau of 2.4-2.0 V, which exceeds the cell voltage of other multivalent-ion batteries, including Zn metal and Al metal batteries. Although the voltage is not yet comparable to commercial lithium-ion batteries, its performance could be boosted by further development.
“The battery serves as a proof-of-concept and demonstrates for the first time the long-term cyclability of an aqueous Mg metal battery,” said Professor Leung.
Link to the research article: https://doi.org/10.1021/acsenergylett.2c01255
Source : https://www.hku.hk/press/press-releases/detail/25056.html